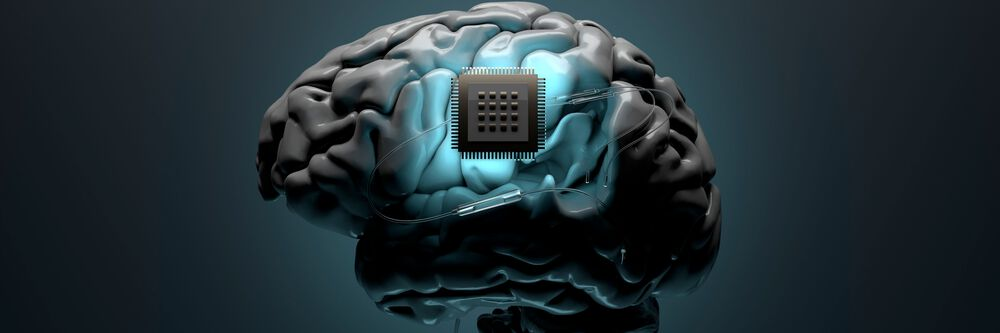
June 2025 — Elon Musk’s Neuralink, the pioneering neurotechnology firm developing advanced brain-computer interface (BCI) systems, has announced a successful Series E funding round totaling $650 million. The round attracted a roster of high-profile investors, including ARK Invest, Founders Fund, DFJ Growth, Gigafund, and Valor Equity Partners, among others. This substantial capital infusion marks a significant milestone in Neuralink’s mission to revolutionize the intersection of neuroscience and computing.
Founded in 2016, Neuralink aims to create implantable devices that connect the human brain to computers, enabling direct communication between neurons and machines. With this fresh capital, the company plans to accelerate clinical trials, expand its engineering and neuroscience teams, and push the development of commercial BCI systems into more advanced stages.
A Vision Beyond the Horizon
Neuralink has long captivated the tech and science communities with its ambitious goals. The technology, still in its nascent stages, has the potential to enable quadriplegics to control computers with their thoughts, treat neurological disorders such as Parkinson’s disease, and eventually create symbiotic relationships between humans and Artificial Intelligence.
While the neural lace—a term Musk coined early on—is still far from mass adoption, this new round of funding is a bold declaration that investors believe in Neuralink’s long-term vision. Elon Musk has previously stated that one of Neuralink’s eventual aims is to mitigate the existential risk posed by superintelligent AI by enabling a tighter integration between humans and machines.
For now, the focus remains on short-term practical applications, particularly restoring mobility and communication abilities to patients with severe spinal cord injuries or neurological damage.
Funding Details and Key Investors
Neuralink’s $650 million Series E round is one of the largest in the field of neurotechnology. The round was led by Founders Fund and Gigafund, both known for their long-term support of Musk-led ventures. Also participating were Google Ventures, Bluesky Capital, Future Ventures, and existing backers like DFJ Growth and ARK Invest.
ARK Invest, an asset manager renowned for its focus on tech news and disruptive innovation, has previously included Neuralink in its speculative growth models. The firm sees Neuralink as part of a broader thesis around human augmentation and the evolution of communication technologies.
In an official statement, Neuralink said, “This latest investment will help us further develop our N1 implant and the R1 surgical robot, conduct rigorous clinical trials, and ultimately commercialize the technology to help millions suffering from neurological impairments.”
Recent Progress and Trials
In early 2024, Neuralink made headlines when it received FDA approval for its first human clinical trials. Shortly after, the company implanted its BCI chip into a patient suffering from ALS (amyotrophic lateral sclerosis), enabling him to move a cursor and type messages using only his thoughts.
The company claims that the procedure was minimally invasive and completed within an hour using its robotic surgeon, the R1, which is designed to insert ultra-thin threads into the brain without damaging blood vessels.
The implanted chip, called the N1 Link, can read and transmit brain activity via Bluetooth to an external device. Future iterations are expected to include wireless charging, higher signal resolution, and biocompatibility enhancements to ensure long-term safety.
Neuralink has also begun expanding its recruitment for participants in subsequent trial phases and is working closely with neurologists, engineers, and ethicists to ensure the trials adhere to rigorous scientific and ethical standards.
Implications for Neurotechnology and AI
The potential applications of Neuralink’s BCI span across various domains. Beyond medical treatments, Neuralink’s technology could revolutionize human interaction with AI systems, leading to seamless brain-to-machine communication.
As brain data becomes more accessible and machine learning models more advanced, the convergence of neurotechnology and Artificial Intelligence could yield tools capable of interpreting and responding to human thoughts in real-time. This fusion could lead to new AI tools for diagnosing mental health conditions, optimizing cognitive performance, or even enabling telepathic communication.
A link to one of these keywords — Artificial Intelligence — illustrates how deeply connected Neuralink’s objectives are to broader developments in the AI and tech ecosystem. The boundaries between human cognition and digital processing are blurring, offering both profound potential and serious ethical challenges.
Ethical, Regulatory, and Cybersecurity Challenges

As Neuralink moves toward commercialization, it must navigate a complex regulatory landscape. Neuroethics experts have raised concerns about consent, privacy, and the psychological effects of long-term BCI use.
Moreover, integrating human brains with digital systems inevitably raises cybersecurity concerns. The ability to read or even potentially influence neural activity introduces risks that must be thoroughly addressed. Safeguarding brain data against tampering, unauthorized access, or surveillance will require new layers of encryption and regulation.
The FDA’s involvement helps ensure safety protocols are upheld, but global standards for BCI technologies are still evolving. Initiatives by the IEEE and WHO are in early stages, and the private sector—including companies like Neuralink—will likely help shape future regulations.
The Race Toward Human-Machine Integration
Neuralink is not alone in the race to develop commercial BCI technology. Competitors like Synchron, Blackrock Neurotech, and Paradromics have also made significant progress. Synchron, in particular, achieved a breakthrough with its Stentrode implant, which can be inserted via blood vessels, reducing surgical complexity.
However, Neuralink’s end-to-end system, which includes both implants and surgical robotics, sets it apart. The company’s strategy of vertical integration—controlling every aspect from chip fabrication to surgical procedure—is reminiscent of Tesla’s model and gives it a competitive advantage.
Industry analysts believe that Neuralink’s substantial Series E funding may ignite a new wave of venture capital interest in neurotech, similar to what occurred with Blockchain and biotech sectors in previous years.
Looking Forward: From Medical Devices to Consumer Gadgets?
While the initial applications of Neuralink’s technology are medical, Elon Musk has hinted at potential consumer applications. Future versions could offer cognitive enhancements, memory backups, or real-time language translation.
“There will come a point where having a BCI might be as normal as wearing a smartwatch,” said Musk during a presentation last year. “The human-computer interface will be seamless, instantaneous, and intuitive.”
This statement hints at a future where BCI devices evolve from niche medical implants to mainstream gadgets. Yet such a vision will require monumental advances in hardware, user safety, data integrity, and public trust.
Conclusion: A Historic Inflection Point
Neuralink’s $650 million Series E round is more than just another funding announcement—it marks a historic inflection point in the journey toward human-machine symbiosis. The capital not only boosts the company’s R&D capabilities but also affirms the growing investor confidence in neurotechnology as a transformative industry.
With foundational support from some of Silicon Valley’s most influential investment firms, Neuralink is poised to become a central player in shaping the future of brain-computer interfaces. Its success could redefine how we treat neurological diseases, interact with digital systems, and even understand human consciousness.
Yet, as with all groundbreaking technologies, the path forward will be shaped by a balance of innovation, regulation, and ethics. Neuralink stands at the forefront of a revolution that could rival the advent of smartphones, the internet, and even Machine Learning in its societal impact.
The years ahead will reveal whether Neuralink can translate its vision into real-world breakthroughs. One thing is certain: the world is watching, and the future of neurotechnology has never looked more electrifying.
Related Reading and Sources:
- Neuralink’s Clinical Trial Updates – FDA Approval and Results
- Neuroethics Concerns and Brain Data Privacy
- The Rise of Neurotechnology: Industry Report by McKinsey & Company
- Synchron vs Neuralink: The Neurotech Battle
- Web3 and Neurotech: Future Frontiers